Syllabus Link
UPSC Mains GS II: Issues relating to development and management of Social Sector/Services relating to Health; Indian Constitution- fundamental rights.
This is a high-yield topic for GS Paper II (Social Justice/Health/Constitution) and GS Paper IV (Ethics – Corporate Governance/Compassion). The convergence of judicial activism in mental health and executive strictness in pharmaceutical standards marks a pivotal shift in India’s public health landscape
Context: The Supreme Court ruled that mental health is a fundamental right under Article 21, and the Health Ministry mandated strict compliance with revised Schedule M norms following Diethylene Glycol (DEG) contamination incidents in cough syrups.
Introduction
India’s health sector is currently witnessing a dual transformation. On one hand, the Supreme Court has expanded the “Right to Life” (Article 21) to explicitly include mental health, moving it from a welfare concern to a constitutional guarantee. On the other hand, the “Pharmacy of the World” is undergoing a rigorous quality overhaul through the Revised Schedule M norms to combat the menace of substandard drugs (specifically Diethylene Glycol contamination). Together, these developments signify a shift towards a rights-based and quality-assured health framework.
Historical Evolution & Background
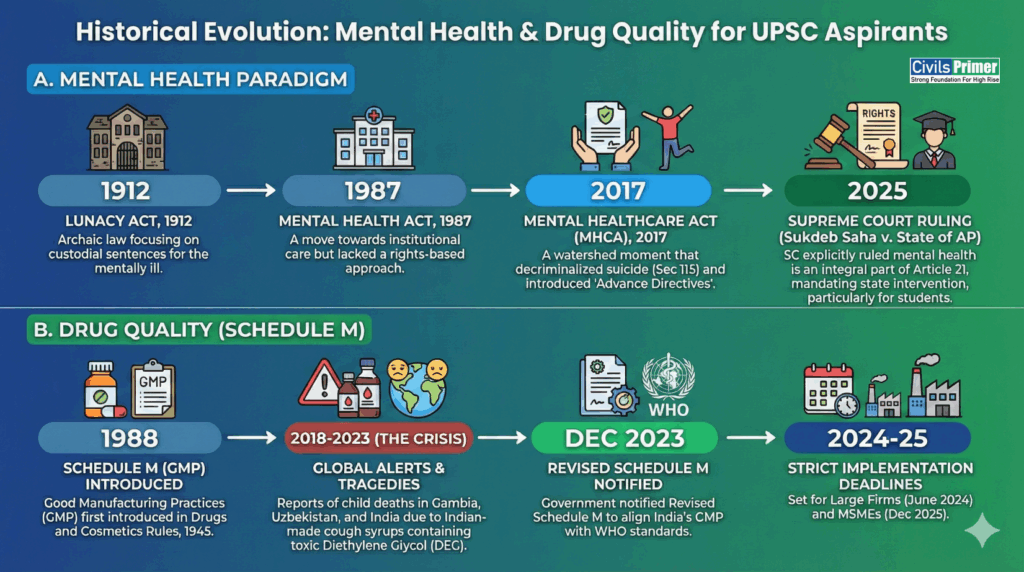
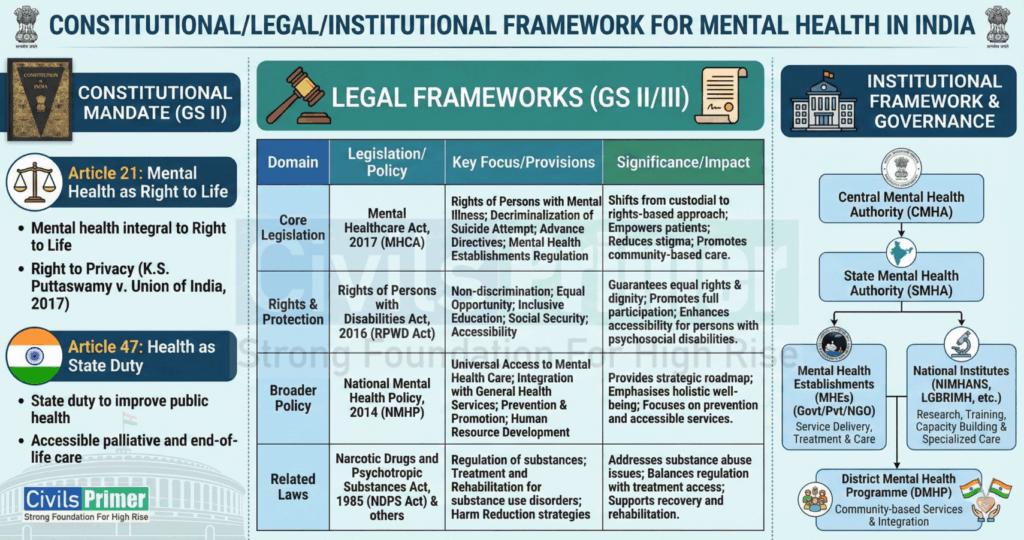
Constitutional & Institutional Framework
| Dimension | Mental Health | Pharmaceutical Quality (Schedule M) |
| Constitutional | Article 21: Right to Life & Dignity. Article 47: State duty to improve public health. | Article 21: Right to Health (Safe Drugs). Article 19(1)(g): Right to trade, subject to reasonable restrictions (public safety). |
| Legal | Mental Healthcare Act, 2017: – Sec 21: Right to Equality/Non-discrimination. – Sec 115: Decriminalization of suicide attempts. | Drugs and Cosmetics Act, 1940: Schedule M: Good Manufacturing Practices (GMP). Sec 27: Penalties for spurious drugs. |
| Institutional | Central/State Mental Health Authorities (SMHA). Kiran Helpline & Tele-MANAS. | CDSCO (Central Drugs Standard Control Org). State Drug Controllers. |
Case Studies (For Mains Value Addition)
Case Study 1: The “Coldrif” & “Maiden Pharma” Tragedies (Pharma Crisis)
- Incident: Deaths of children in Gambia (Maiden Pharma) and Madhya Pradesh (Coldrif Syrup) were linked to Diethylene Glycol (DEG). DEG is a toxic industrial solvent used illegally as a cheap substitute for Propylene Glycol.
- Why it matters: It exposed the “weak link” in India’s supply chain—MSMEs often lack testing facilities for raw materials.
- Lesson: Quality control cannot be retrospective; it must be embedded in the manufacturing process (Quality by Design).
Case Study 2: Sukdeb Saha v. State of Andhra Pradesh (Mental Health)
- Context: Following the suicide of a 17-year-old aspirant, the SC took cognizance of the mental pressure in coaching hubs.
- Ruling: Mental health is a fundamental right.
- Outcome (Saha Guidelines): Mandatory counseling units for institutions with >100 students; 24/7 helplines; and “de-stigmatization” protocols.
Government Initiatives (Domestic)
Strengthening Drug Quality (Revised Schedule M)
The revised norms mandate five key changes (Keywords for UPSC Mains Answers):
- Pharmaceutical Quality System (PQS): Moving from “Quality Control” (testing at end) to “Quality Assurance” (checking every step).
- Quality Risk Management (QRM): Proactive identification of contamination risks.
- Product Quality Review (PQR): Annual review of drug consistency.
- Computerized Storage Systems: To prevent data manipulation (ensuring data integrity).
- Equipment Validation: Mandatory validation of all machinery.
- PTUAS Scheme: Pharmaceutical Technology Upgradation Assistance Scheme provides subsidies/loans to MSMEs to upgrade to Schedule M standards.
Mental Health Support
- Tele-MANAS: A 24/7 national tele-mental health programme launched to boost access.
- Manodarpan: Initiative by Ministry of Education for psychosocial support to students.
- Ayushman Bharat: Now covers mental health treatment packages.
Issues, Challenges & Gaps (Prioritize for Mains)
- The “MSME Burden” (Pharma): Upgrading to Revised Schedule M costs ₹50 Lakh to ₹2 Crore. About 80% of India’s 10,500 drug units are MSMEs; they lack the capital and may shut down, impacting drug availability.
- Dual Regulation Structure: Health is a state subject. The CDSCO (Centre) sets standards, but State Drug Controllers (SDCs) issue licenses. This leads to regulatory arbitrage (manufacturers moving to states with lax enforcement).
- Manpower Deficit (Mental Health): India has 0.75 psychiatrists per 100,000 population . The implementation of Article 21 is impossible without infrastructure.
- Invisible “DEG” Threat: Testing for Diethylene Glycol requires Gas Chromatography (expensive equipment), which small firms do not possess.
- Insurance Exclusions: Despite IRDAI mandates, many insurers still reject mental health claims, violating the “Right to Equality.”
- Social Stigma: 80% of people with mental disorders in India do not seek treatment due to stigma (The “Treatment Gap“).
Stakeholders & Their Roles
| Stakeholder | Role and Responsibility |
|---|---|
| Supreme Court / Judiciary | Interprets Article 21 to expand rights (mental health, dignity); issues binding orders (Saha Guidelines) and ensures institutional accountability. |
| Health Ministry (MoHFW) / CDSCO | Designs and implements national health missions (PM-ABHIM); enforces Schedule M norms; manages drug licensing via ONDLS. |
| Pharmaceutical Industry | Mandatory adoption of Pharmaceutical Quality System (PQS) and ALCOA+ Data Integrity principles under revised Schedule M. |
| Educational Institutions | Required to implement supportive mental health systems and counseling in compliance with Saha Guidelines. |
| Civil Society & NGOs | Provide peer counselling, community support, and advocacy to help reduce social stigma and facilitate access to care |
Way Forward & Visionary Recommendations, Reform Areas
- Institutional Accountability (Drugs):
- Move towards a Single National Drug Regulator (unifying State and Central controls) to ensure uniform enforcement of Schedule M. Ensure strict digital monitoring of high-risk solvents (like DEG) through the ONDLS portal. Implement mandatory ethics CME and blacklist convicted providers to deter medical complicity in fraudulent manufacturing. Create a “National Digital Drugs Registry” to track raw material batches (e.g., Propylene Glycol) from import to factory floor to prevent DEG contamination.
- Legal Fix & MHA Compliance for Mental Health: Mandatory establishment and full staffing of Mental Health Review Boards (MHRB) in all districts within a one-year timeline. This ensures decentralized legal oversight and rights enforcement for patients
- Financial Support: Expand the PLI Scheme to include financial aid for MSMEs specifically for Quality Upgradation (Compliance-linked incentives). Increase mental health allocation from 1% to 3–5% of the total health budget. Funds should be specifically directed toward community-based care and school-level support systems
- Curriculum Integration: As per SC guidelines, mental health “First Aid” should be a mandatory part of school/college curricula, not just an annexure.
- Community-Based Care: Shift focus from “Institutional Care” (Asylums) to “Community Care” (Half-way homes) to handle the patient load. Launch a National Geriatric and Mental Health Cadre expansion programme to train specialized doctors and nurses, aiming to meet the WHO psychiatrist ratio within five years.
- Awareness & Stigma Reduction: Implement national, state-led anti-stigma campaigns (e.g., using digital media and local influencers) and promote peer networks to normalize help-seeking behavior and build social resilience
Prelims Traps
| Fact/Term | Details (Revision Notes) | Likely Trap |
| Revised Schedule M | Deals with GMP (Good Manufacturing Practices). Mandatory for ALL drug manufacturers. Schedule M is a part of the Drugs and Cosmetics Act, 1940. The CDSCO (Central Drugs Standard Control Organisation) is the apex regulatory body enforcing these norms. India supplies 20% of global generic medicines. | Confusing it with Schedule H (Prescription drugs) or Schedule X (Narcotics). |
| Diethylene Glycol (DEG) | Toxic industrial solvent. Causes Acute Kidney Injury (AKI). Used to fake Glycerin/Propylene Glycol. | Thinking it is a bacterial contaminant (It is a chemical adulterant). |
| Article 21 Scope | Now explicitly includes Mental Health (Right to Life with Dignity). | Thinking Mental Health falls only under DPSP (Article 47). |
| Sec 115 (MHCA 2017) | Decriminalizes suicide attempts due to severe stress. | Trap: “It repeals Sec 309 IPC.” (No, it restricts its application; Sec 309 technically remains in IPC). |
| CDSCO | Headed by DCGI. Under Min. of Health & Family Welfare. | Thinking it is a statutory body under NITI Aayog. |
| Tele-MANAS | National Tele-Mental Health Programme. | Thinking it is an app for physical fitness. |
Quick Facts: Mental Health Care in India
- India ranks 3rd worldwide in pharmaceutical production by volume and supplies 20% of global generic medicines. India is a significant vaccine contributor, sourcing 55–60% of UNICEF’s vaccines
- The MHA 2017 mandates the establishment of Mental Health Review Boards (MHRB) in every state. India still faces a severe shortage of 0.75 psychiatrists per 100,000 people (WHO norm: 3)
Previous Year Questions (PYQs)
- Mains 2023 (GS II): “Appropriate local community-level healthcare intervention is a prerequisite to achieve ‘Health for All’ in India. Explain.” (Use Mental Health community models here)
- Mains 2020 (GS II): “In order to enhance the quality of democracy in India, the Election Commission of India has proposed electoral reforms in 2016. What are the suggested reforms and how far are they significant to make democracy successful?” (While this is Polity, the structure of “Reforms -> Implementation” applies to Drug Regulation too).
- Mains 2013 (GS II): “Occupational safety and health… Discuss the resilience of the legal framework.” (Relate to Industrial safety in Pharma manufacturing).
- Prelims 2019: Question on Rights of Persons with Disabilities Act, 2016 (Related to mental illness inclusion).
